Effect of boron oxide on the
structure and optical properties of magnesium fluoride
Chem.
Met. Alloys 16 (2023)
1-6
Viktor ZINCHENKO, Igor MAGUNOV, Anton BABENKO, Ganna
VOLCHAK, Serhii KULESHOV, Oleksandr IVANENKO
https://doi.org/10.30970/cma16.0427
Magnesium fluoride,
MgF2, is a widely used compound in electrical engineering,
optoelectronics, etc. Materials based on it have high transparency up to
the deep (vacuum) ultraviolet range of the spectrum. Their main disadvantage is
the presence of oxide impurities, mainly MgO, which appear during heat
treatment and prolonged storage in open air due to hydrolysis or incomplete
reaction during synthesis. Addition of boron oxide, B2O3,
is proposed to bind oxide impurities of basic nature into complex compounds
such as fluoroborates. The amount of additive was calculated based on the
estimated content of MgO impurities in the material. Excess B2O3
was washed with ethanol, which was then removed by prolonged heat treatment.
The base substance, MgF2, and reaction products were identified by
infrared transmission spectroscopy and X-ray diffraction. The IR transmittance
spectra of the original sample clearly show the bands characteristic of MgF2,
and after the addition of the additive, absorption bands characteristic of B–O
bonds are detected, the intensity of which decreases when the additive is
washed away. The original MgF2 preparate is single-phase, but the
increase in the width of the peaks and their shift indicate a disturbance in
the structure. The average size of the MgF2 crystallites was
calculated according to the well-known Scherrer equation, and
is in the range of 14–20 nm (average value 18 nm). After heat treatment with
the addition of B2O3, the width of the reflections
decreases significantly, and after subsequent washing and calcination, the
diffractogram of the sample reveals the reflections of magnesium fluoborite, Mg3(BO3)F3. According to a quantitative
analysis by the Rietveld method, its content is about 8.7% by volume. The
thickness of the MgO surface layer on the surface of MgF2-particles
was calculated (about 0.068 nm), and confirms the
X-ray amorphous nature of the impurity.
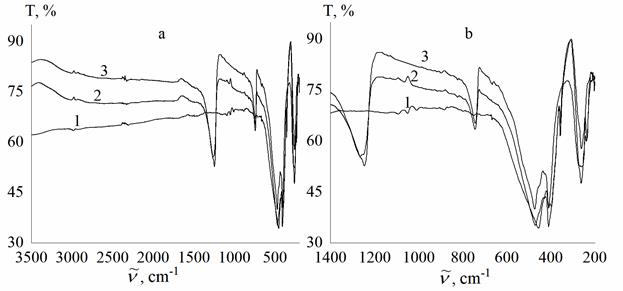
Infrared transmission spectra in the intervals
3500-200 (a) and 1400-200 (b) cm-1 of MgF2 samples: 1 –
original sample, 2 – sample after heat treatment with B2O3,
3 – sample after heat treatment with B2O3, removal of
excess B2O3 and repeated heat treatment.
Keywords
Magnesium fluoride /
Oxide impurity / Boron oxide / Infrared spectroscopy / X-ray phase analysis