Activation of thin layers of two aromatic hydrocarbons
Chem.
Met. Alloys 4 (2011) 31-37
https://doi.org/10.30970/cma4.0142
Sylwester KANIA, Janusz KULIŃSKI
The kinetics of activation currents induced by
ethanol into thin layers of two aromatic hydrocarbons were
investigated. The results indicate a partial chemisorption
process with injection of carriers. Assuming that the increase of the current
over the initial value is proportional to the covered surface, the enthalpies
determined from the slope of the C2H5OH adsorption
dependence were nearly identical for tetracene and
for p‑quaterphenyl. This suggests a similar
mechanism for the effect of adsorption on the electron structure of the
adsorbing molecule for both of the substances. Variations of the conductivity
were observed due to the adsorption of ethanol, which suggests that hopping
through localized states is the dominant mode of charge transport.
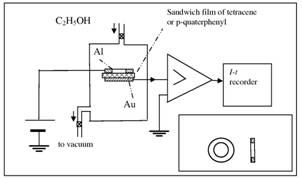
Experimental set-up for activating
the films and for recording the I-t
characteristics of the dark current induced by the activation process. Insert shows the construction of
the upper aluminium electrode.
Keywords
Charge transport / Acenes
/ Adsorption / Tetracene / p‑quaterphenyl