Copper(I) complexes with 5-(allylthio)-1H-tetrazoles:
synthesis and crystal structure of [Cu2(C10H10N4S)2(H2O)2](BF4)2
and [Cu2(C10H9ClN4S)2(H2O)2](BF4)2×C2H5OH π-compounds (C10H10N4S
and C10H9ClN4S - 5-(allylthio)-1-phenyl-
and 5-(allylthio)-1-(4-chlorophenyl)-1H-tetrazole)
Chem.
Met. Alloys 3
(2010) 201-207
https://doi.org/10.30970/cma3.0170
Yuriy SLYVKA, Nazariy
POKHODYLO, Roman SAVKA, Zoran MAZEJ, Evgeny
GORESHNIK, Maryan MYS’KIV
By means of the alternating current electrochemical
technique, [Cu2(atpt)2(H2O)2](BF4)2
(1) (atpt = 5-(allylthio)-1-phenyl-1H-tetrazole,
C10H10N4S) and [Cu2(atcpt)2(H2O)2](BF4)2×C2H5OH (2) (atcpt = 5-(allylthio)-1-(4-chlorophenyl)-1H-tetrazole,
C10H9ClN4S) π-complexes have been synthesized
and X-ray studied by the single crystal method. Crystals of 1 are monoclinic, space group P21/n, a =
10.4353(7), b = 10.9125(7), c = 12.9389(9) Å, β = 99.064(2)°, V =
1455.02(17) Å3 at 200 K, Z = 2, R =
0.067 for 2881 reflections; compound 2 crystallizes in the triclinic space group ![]() , a =
10.925(2), b = 11.945(2), c =
15.168(3) Å, α = 75.89(1), β = 86.23(1), γ =
64.13(1)°, V = 1725.4(4) Å3 at 298 K, Z = 2, R = 0.066 for 2590 reflections. In
these structures the metal atoms possess a trigonal
pyramidal coordination environment involving N3 and N4 atoms from two adjacent
organic ligands, an olefin C=C bond and a water
molecule in apical position. Both the atpt and
atcpt ligand
molecules act as a tridentate N,N,(S-C3H5)-bridging
ligand connecting two metal (M) atoms into centrosymmetric cationic [M2(L)2(H2O)2]2+ dimers with one six-membered {M2N4} cycle and two seven-membered {MNC4S} rings. Effective O-H...F bonds occur between
the coordinated water molecules and the BF4– anion.
, a =
10.925(2), b = 11.945(2), c =
15.168(3) Å, α = 75.89(1), β = 86.23(1), γ =
64.13(1)°, V = 1725.4(4) Å3 at 298 K, Z = 2, R = 0.066 for 2590 reflections. In
these structures the metal atoms possess a trigonal
pyramidal coordination environment involving N3 and N4 atoms from two adjacent
organic ligands, an olefin C=C bond and a water
molecule in apical position. Both the atpt and
atcpt ligand
molecules act as a tridentate N,N,(S-C3H5)-bridging
ligand connecting two metal (M) atoms into centrosymmetric cationic [M2(L)2(H2O)2]2+ dimers with one six-membered {M2N4} cycle and two seven-membered {MNC4S} rings. Effective O-H...F bonds occur between
the coordinated water molecules and the BF4– anion.
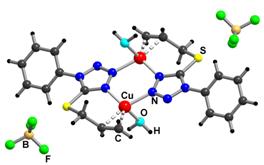
Fragment of [Cu2(C10H10N4S)2(H2O)2](BF4)2 crystal structure
Keywords
Tetrazole / Copper(I) / Electrochemical
technique / π-Complex
/ Crystal structure