“Post-hydrogenation”
structural transformations from KHg2- to Fe2P-type in R(Cu,Ni)2 hydrides (R = Ce, Pr, Nd)
Chem.
Met. Alloys 3
(2010) 169-176
https://doi.org/10.30970/cma3.0155
A.B. RIABOV, R.V. DENYS, R. ČERNÝ, I.Yu. ZAVALIY
Hydrogenation of orthorhombic KHg2-type
R(Cu,Ni)2 pseudobinary intermetallic compounds (R = Ce, Pr, Nd)
was found to lead to the formation of an orthorhombic insertion-type hydride,
which readily transforms into a hexagonal Fe2P-type hydride. This
“post-hydrogenation” structural transformation can be accelerated by heating in
vacuum at 100-120°C and is accompanied by release of
¼ of the available hydrogen, as has been confirmed by PND studies. Further heating in vacuum leads to disproportionation of the hydride and recombination with
recovery of the orthorhombic R(Cu,Ni)2 compound
after complete elimination of hydrogen from the material.
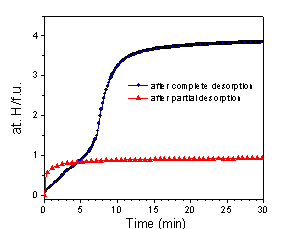
Repeated hydrogen absorption by Pr(Cu0.75Ni0.25)2 after complete recombination during desorption, and after partial hydrogen release up to 120°C.
Keywords
Metal hydrides /
Crystal structure / X-ray diffraction / Powder neutron diffraction