Solid solutions with AlB2-type structure in R-Ag-Al-Ge systems (R
= Ce, Pr, Nd, Sm)
Chem.
Met. Alloys 2
(2009) 157-163
https://doi.org/10.30970/cma2.0113
Roksolana KOZAK, Yaroslav
TOKAYCHUK, Mykola MANYAKO, Roman GLADYSHEVSKII
An investigation of the quaternary systems R-Ag-Al-Ge (R = Ce, Pr, Nd, Sm) at 873 K led to
the discovery of three complete solid solutions based on ternary germanides, CeAg0.8Ge1.2-CeAl1.6-1.5Ge0.4-0.5, PrAg0.8Ge1.2-PrAl1.55-1.48Ge0.45-0.52,
and NdAg0.7Ge1.3-NdAl1.63-1.50Ge0.37-0.50, and a quaternary compound, SmAg0.55-0.36Al0.43-0.80Ge1.02-0.84, with AlB2-type
structure (Pearson symbol hP3, space group P6/mmm). The crystal structure of the solid
solution in the system Pr-Ag-Al-Ge was refined for the composition PrAg0.38Al0.80Ge0.82 from X-ray powder (a = 0.43368(5), c = 0.41929(7) nm) and single-crystal (a = 0.4318(1), c = 0.4191(1) nm) diffraction data. Within the homogeneity range the contact distances between
small-size atoms decrease with increasing Al and
decreasing Ag and Ge content. The valence electron concentration per atom of
the statistical mixture Ag+Al+Ge increases from 4.30 to 4.76 within the same range.
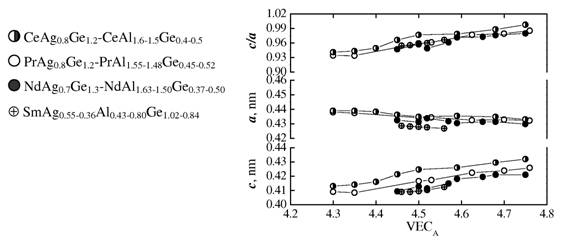
Unit-cell parameters vs. valence electron concentration within the homogeneity ranges of the AlB2-type
compounds in the
quaternary systems {Ce,Pr,Nd,Sm}-Ag-Al-Ge.
Keywords
Rare-earth / Silver / Aluminum / Germanium /
Solid solution / X-ray diffraction / Crystal structure