Corrosion protection
of mild steel electrode by electrochemical polymerization of acrylamide
Chem.
Met. Alloys 2
(2009) 202-210
https://doi.org/10.30970/cma2.0108
Polyacrylamide (PAA) film was electrosynthesized
on mild steel by cyclic voltammetry using a Ce(IV)
salt – oxalic acid initiator system. Polymerization was initiated by a free
radical that is formed by fast reaction of oxalic acid and Ce(IV). The electrolysis of
the solution results in regeneration of Ce(IV), which can oxidize the oxalic acid and produce
radicals. The effect of temperature on the yield
of the electroinitiated polymerization
was studied. The potential sweep rate was changed to achieve
polymer films with different levels of thickness. The capacity of the PAA film
to protect mild steel from corrosion in an 1 M NaCl aqueous solution was investigated by potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS). The structure of the
PAA film on mild steel was investigated by physicochemical methods such as
elemental analysis of C, H and N by a FTIR spectrometer. The results of
the studies reveal that the corrosion resistance of PAA-coated mild steel is
significantly higher and the corrosion rate considerably lower than for
uncoated steel. PAA films formed with lower sweep rates exhibited a larger
positive shift of the corrosion potential and greater charge transfer
resistance, reflecting as higher inhibitors for corrosion of mild steel.
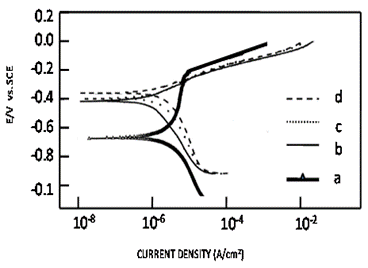
Potentiodynamic polarization curves
for uncoated mild steel and;
PAA coated on mild steel in
aqueous 1M NaCl solution.
Keywords
Polyacrylamide / Electrochemical / Potentiodynamic / Impedance / Protection / Corrosion