Polarographic investigation of reduction processes of gallium(III) complexes with some o,o'-dihydroxysubstituted
azo dyes
Chem.
Met. Alloys 2
(2009) 194-201
https://doi.org/10.30970/cma2.0107
Solomiya PYSAREVSKA, Liliya DUBENSKA, Nataliya SHAJNOGA, Halyna
LEVYTSKA
The voltammetric behaviour of Ga(III) complexes with o,o'-dihydroxysubstituted
azo dyes (eriochrome red B
(ERB), eriochrome black T (EBT), calcon
(Calc), and kalces (Klc))
was investigated using cyclic linear-sweep voltammetry.
Two new reduction peaks caused by reduction of the Ga(III)–azo
dye complex compounds were registered on the voltammograms.
The effect of pH, the concentration of azo dye and Ga(III)
ions, and the sweep rate on the peak current was studied. The peak current
changed linearly with increasing gallium concentration. New methods were
proposed for the determination of metal ions. The detection limits were
2.0×10‑7 M for the Ga(III)–ERB
complex, 1.0×10‑6 M for the Ga(III)–EBT
and Ga(III)–Calc systems, 5.3×10‑7 M
for the Ga(III)–Klc complex
based on the first peak and 9.1×10‑7 M for the same
system but based on the second peak. The proposed methods were tested on the
determination of gallium in intermetallic compounds
of the Zn–Ga and Sm–Ga systems and in a Gd3Sc2Ga3O
luminophore.
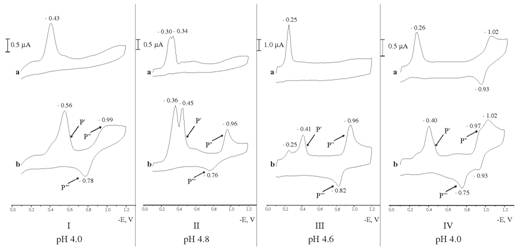
Cyclic voltammograms of 4×10-5 Ì (²à) ERB, (²²à) EBT, (²²²à) Klc, (IVà) Calc and (²b) ERB–Ga(III), (IIb) EBT–Ga(III), (IIIb) Klc–Ga(III), (IVb) Calc–Ga(III) complexes, CGa(III)=Cazo dye=4×10-5 Ì.
Keywords
Gallium / Azo dye / Voltammetry / Reduction / Dropping mercury electrode