Synthesis and crystal
structure of copper complexes with N,N,N,N',N' – pentaallylethylenediammonium:
[(C3H5)3NC2H4N(H+)(C3H5)2]CuIICl4
and [(C3H5)3NC2H4N(H+)(C3H5)2]CuIBr3
Chem.
Met. Alloys 2
(2009) 138-145
https://doi.org/10.30970/cma2.0103
Mykhaylo MONCHAK, Evgeny GORESHNIK, Volodymyr
DAVYDOV, Maryan MYS’KIV
N,N,N,N',N'-pentaallylethylenediammonium
has been obtained by reaction of ethylenediamine and allyl bromide in benzene +ethanol (1:5) medium in the presence of NaHCO3. After 48 hours of mixing
and boiling the mixture was filtered. Good quality crystals of the complexes
[(C3H5)3NC2H4N(H+)(C3H5)2]CuIICl4 (I)
and [(C3H5)3NC2H4N(H+)(C3H5)2]CuIBr3 (II)
were obtained using the alternating-current electrochemical technique and then
X-ray investigated. The crystal structures of I and II are monoclinic, for I:
space group P21,
a = 7.596(2),
b = 18.302(6),
c = 8.271(3) Å,
β = 103.80(2)°,
V = 1116.7(6) Å3,
Z = 2; for
II: space group P21/c, a =
8.328(1),
b = 18.377(3),
c = 13.854(2) Å,
β = 95.31(1)°,
V = 2111.1(5) Å3,
Z = 4. In
both structures simple (CuCl42– and CuBr32–
respectively) anions occur. Due to the absence of π-interaction in I and
II the organic and inorganic moieties are held together by electrostatic
interaction and E–H…X (E =
N, C; X = Cl, Br) bonds.
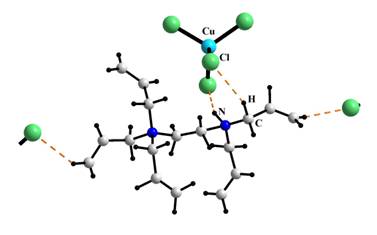
Fragment of I crystal structure
Keywords
Copper complexes / Ethylenediamine / N,N,N,N',N'-pentaallylethylenediammonium
bromide / Isolated inorganic anions