Products of the interaction of copper and benzenediazonium
tetrafluoroborate in acetonitrile medium
Chem.
Met. Alloys 2
(2009) 123-129
https://doi.org/10.30970/cma2.0099
Eugen P. Koval’chuk,
Oleksandr V. Reshetnyak, Vasyl’
Ya. Smetanets’kyj, Jerzy Błażejowski
The products of the chemical interaction of
copper and a concentrated (³0.05 M) benzenediazonium
tetrafluoroborate (BDFB) solution in acetonitrile have been studied. It is
proposed on the base of the obtained results that the intermediate of the
copper dissolution-ionization, which corresponds to an absorption band with a
maximum at 432 nm, is the mixed complex [Cu(Nº
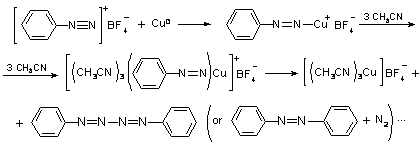
Mechanism of DAS - Cu interaction in the acetonitrile
medium
Keywords
Ñopper / benzenediazonium
tetrafluoroborate / acetonitrile
/ dissolution-ionization / Cu(I)
complexes /