Crystal structure of the complex K4[W2(CN)10O3]∙4H2O
Chem.
Met. Alloys 2
(2009) 25-29
https://doi.org/10.30970/cma2.0082
Iryna TYPILO, Olha SEREDA, Helen
STOECKLI-EVANS, Dariya
SEMENYSHYN,
Roman GLADYSHEVSKII
The complex K4[W2(CN)10O3]∙4H2O
was prepared by hydrolysis of K4[W(CN)8]∙2H2O
and its crystal structure solved by direct methods. Crystal data: C10H8K4N10O7W2,
Mr = 904.34, triclinic
system, space group P1, cell parameters a = 8.7910(8), b =
8.8424(8), c =
9.4743(9) Å, α =
82.316(11), β = 64.843(10),
γ = 64.945(10)º, V = 602.92(10) Å3,
Ζ = 1, DX = 2.491 g cm-3, R =
0.0236 and wR = 0.0584 for 2175 independent reflections. The
coordination polyhedra of the tungsten(VI) atoms are
pentagonal bipyramids W(CN)5O2, which are linked into
pairs by sharing one of the oxygen atoms and twisted by 36º with respect
to each other. The hydrogen bonds interconnect the pairs of pentagonal
bipyramids into infinite complex chains. The
coordination polyhedra of the two independent potassium atoms are
trigonal prisms with one capped face (by a NC unit), but have different
composition, K(1)(NC)4O(OH2)2 and K(2)(NC)5(OH2)2.
The formula of this coordination compound can be written as K4[OW(CN)5OW(CN)5O]·4H2O.

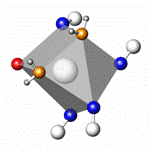
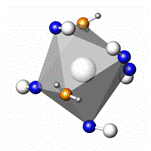
W2(CN)10O3 K(1)(NC)4O(OH2)2 K(2)(NC)5(OH2)2
Coordination polyhedra of the W and K atoms in
K4[W2(CN)10O3]·4H2O.
Keywords
Tungsten(VI) complex / Crystal structure / Coordination cyanide


