Synthesis and crystal structure of the complexes of
cobalt(II) and nickel(II) with cyclohexyl acetoacetate and pyridine
Chem.
Met. Alloys 14 (2021)
38-42
https://doi.org/10.30970/cma14.0411
Olherd SHTOKVYSH, Lyudmila
KOVAL, Viktoriya DYAKONENKO, Boris KHOMENKO, Vasyl PEKHNYO
Intracomplex compounds
of Co2+ and Ni2+ with cyclohexyl
acetoacetate and pyridine have been synthesized. The
cobalt complex has been characterized by X-ray diffraction at 129 K and 298 K.
The crystals belong to monoclinic crystal system, space
group I2/a, Z = 4, a =11.8751(12), b = 17.8671(18), c = 14.5630(19) Å, β = 107.859(9)°, V
= 2941.0(6) Å3 (129 K); a = 11.8431(6), b = 18.4288(9), c = 14.5758(11) Å, β = 107.067(2)°, V
= 3041.1(3) Å3 (298 K). The molecules of the complex have a distorted
octahedral environment O4N2 of the central atom with an
axial arrangement of pyridine molecules and equatorial arrangement of chelating
ligands. The bond lengths of the central atom to the
donors are close. The nickel complex is isostructural
to the cobalt complex.
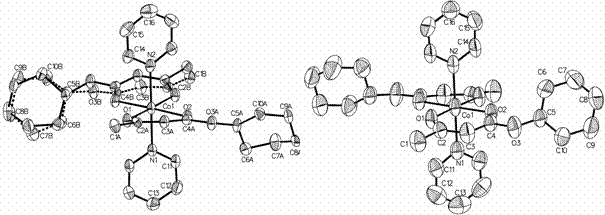
Molecular structure of the complex [Co(C10H15O3)2(C5H5N)2]
at 129 K (left) and 298 Ê (right).
Keywords
Cobalt / Nickel / Cyclohexyl Acetoacetate / Crystal structure / Complex