Formation of nano-sized oxides in the K-Ta-O
system by chemical reaction of Ta metal with KNO3–KOH melts
Chem.
Met. Alloys 1
(2008) 293-297
https://doi.org/10.30970/cma1.0068
Irene V. KOVALENKO, Liudmyla V. CHERNENKO,
Sergei A. KHAINAKOV, Alexander A. ANDRIIKO,
Vladimir I. LISIN
The reaction of Ta metal with
potassium nitrate melts at 600oC has been studied. It was
established that the composition of the reaction product could be varied by an
appropriate choice of the base-acid properties of the melt, which were
regulated by additions of potassium hydroxide. Pure potassium metatantalate KTaO3 is formed when the KOH/KNO3
molar ratio is about 1.2 or slightly higher. A mixture of the metatantalate with tetra- and ditantalate
(K2Ta4O11 and K2Ta2O6)
is formed at lower molar ratios. The content of tetratantalate
increases as the concentration of KOH in the melt
decreases and it becomes the main reaction product in pure nitrate melt. Pure metatantalate was obtained in the form of powders with grain
sizes of about 80 nm. This product possessed ferroelectric properties with a
dielectric constant of 250-300. Such nano-powders
exhibit the properties of a semiconductor, which is not common for
large-crystal materials. The conductivity is 10-5 S cm-1
at room temperature and increases exponentially with increasing temperature.
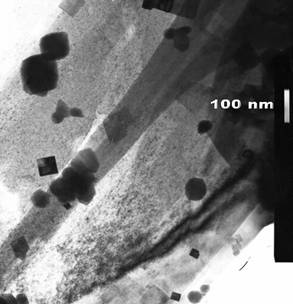
TEM image of the powder of potassium metatantalate.
Keywords
Potassium tantalates
/ Nitrate melts / Nano-sized oxides / Metatantalate / Ferroelectricity