Stable phases of the isothermal (800oC) cross-section of the
pseudo-ternary Li-Mn–Co–O system
Chem.
Met. Alloys 1
(2008) 283-287
https://doi.org/10.30970/cma1.0066
Alexander A.
ANDRIIKO, Arseniy Ye. SHPAK, Nataliya Ye. VLASENKO, Nataliya M. STEPANENKO
The equilibrium phase composition has been
studied in two (pseudo)binary cross-sections of the Li-Mn-Co-O
system at 800°C in ambient air atmosphere: I – LiMn2-xCoxOn (0≤x≤2) and II – LiMnxCo1-xOn (0≤x≤1). Section I
contains a homogeneous spinel-structured phase in the
composition range 0≤x≤1, with the lattice parameter decreasing with
increasing Co content. The compositions become 2-phase for 1<x≤2,
consisting of a Li-deficient spinel structure Ìå3Î4 (Ìå = Ñî, Mn) and
layered LiMeO2 with the
structure of α‑NaFeO2. The compositions in section II are
single-phase only if the Mn/Co atomic ratio does not
exceed ¼ (0≤x≤0.25). Further
increase of the Mn content results in the decomposition
of this phase into three compounds with fixed compositions according to the
overall reaction LiMnxCo1-xOn
→ a LiMn0.25Co0.75O2 + b LiMn1.1Co0.9O4
+ c Li2MnO3. The
phase LiMn0.25Co0.75O2 disappears for 0.6≤x≤1.
Electrochemical testing of single-phase samples from both cross-sections showed
that the replacement of up to 20% of the Mn atoms in
lithium-manganese spinel increases the reversible
capacity of this compound when used as material for the positive electrode of a
Li-ion cell. The electrochemical performance of LiCoO2 deteriorates
with the partial replacement of Co atoms by Mn.
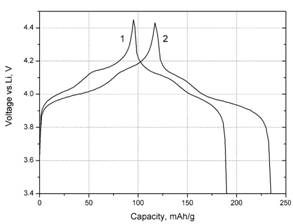
Voltage profiles of galvanostatic
charge and discharge curves for LiMn2O4 (1) and LiMn1.6Co0.4O2
(2) samples.
Keywords
Lithium / Manganese / Cobalt / Oxides / Stable
phases / Electrochemical properties / Li-ion cell