Phase transitions and
formation of polytypes in crystal structures of ionic
compounds
Chem.
Met. Alloys 1
(2008) 235-243
https://doi.org/10.30970/cma1.0036
In the present investigation a new approach for
a design of system of chemical bonds in crystals – geometric conception of
chemical bond (GCCB) – has been used. In GCCB the term “chemical bond”, the shape of the atoms and
the relative dimensions of the valence orbitals of
the atoms are strictly determined in initial postulates that allow to obtain a full collection of all possible valence
configurations of the atoms. The configurations of excited atoms are used as
the elements for design of crystal structures. Models of systems of chemical
bonds in basic structural types of ionic compounds have been created.
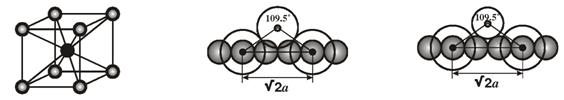
Calculation of the atomic radii in CsCl structures.
Keywords
Ionic radii / Ionic
bond