Crystallization kinetics of the Co77Si11B12 amorphous alloy
Chem.
Met. Alloys 14 (2021)
1-6
https://doi.org/10.30970/cma14.0410
M.M.
LOPACHAK, L.M. BOICHYSHYN, V.K. NOSENKO, B.Ya. KOTUR
The amorphous metal alloy (AMA) Co77Si11B12
obtained by melt-spinning was investigated by X-ray diffraction (XRD) and
differential scanning calorimetry (DSC) at a speed of 5, 10, and 20 K/min. The
DSC curves show three stages of crystallization for all heating rates in the
range of 700-900 K, which are associated with the formation of clusters and
crystalline phases. The first exothermic peak, which indicates the process of nanocrystallization, occurs in the temperature range from
761 to 814 K at different heating rates. Annealing the sample for 1 h at 765 K
caused changes in the XRD profile: the pre-peak at 2θ ≈ 16.5° and
the other three broad peaks at 2θ ≈ 43°, 55° and 82° became sharper, indicating the formation of different
clusters in the amorphous matrix and nanocrystallization
of fcc β-Co
with the lattice parameter a ≈
3.51 Å embedded in the amorphous matrix. The Kissinger method was applied
for calculating the activation energies for the first, second and third DSC
peaks. The activation energy Ea of nucleation and growth of nanocrystals in AMA
Co77Si11B12 are 347 and 374 kJ/mol,
respectively. The S-like form of the
dependences of the volume fraction of the crystalline phase for the first
exothermic peak (α) upon temperature (T)
indicates the predominance of diffusion-controlled processes at high heating
rates. According to the Matusita model, the value of
the growth parameter m showed that
the growth of Co nanocrystals in AMA occurs by a three-dimensional mechanism,
and the value of p = 0.5 indicates a
diffusion-controlled crystallization.
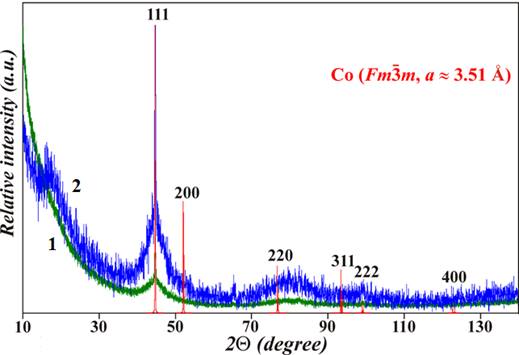
XRD profiles of AMA Co77Si11B12
for the as-quenched sample (green) and for the same sample annealed at 765 K (blue).
Keywords
Amorphous metallic alloys / Nanocrystallization
/ Cobalt / X-ray diffraction / Differential scanning calorimetry