Electrochemical hydrogenation
of the R2Zn17-xMnx phases (R =
Y, La, Gd, Tb)
Chem.
Met. Alloys 13 (2020)
78-84
https://doi.org/10.30970/cma13.0409
Nataliya Chorna, Vasyl KORDAN, Vitalii Nytka, Oksana Zelinska,
Ivan TARASIUK, Anatoliy Zelinskiy,
Volodymyr PAVLYUK
The electrochemical
hydrogenation of the solid solutions R2Zn17-xMnx, x = 0.5-0.9
(R = Y, La, Gd, Tb) was
studied in Ni-MH battery prototypes for the first time. Phase analysis of the
electrode materials on the basis of these compounds
was carried out before and after hydrogenation by X-ray powder diffraction,
scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray
fluorescence spectroscopy. All the majority phases crystallize in the hexagonal
Th2Ni17-type structure. As electrodes the alloys showed similar
electrochemical behavior and the amount of deintercalated hydrogen increased in
the order La < Y < Gd < Tb. The largest
degree of amorphization and formation of oxides, such as ZnO
and R2O3, were
observed for the Y- and La-containing alloys after 50 cycles of hydrogenation/dehydrogenation.
The geometrically most advantageous sites are octahedral voids in Wyckoff
position 6h of the initial structures
with a coordination polyhedron [HR2(Zn,Mn)4] for the H-atom.
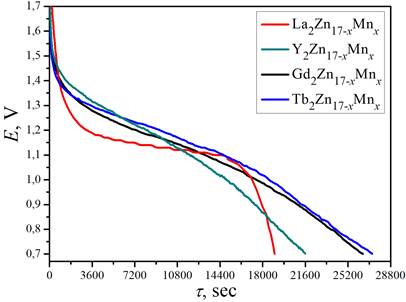
Selected
discharge curves for the battery prototypes based on R2Zn17-xMnx.
Keywords
Solid solution / Th2Ni17-type
structure / Electrochemical hydrogenation / Ni-MH battery