Electrochemical hydrogenation
of solid solutions based on the intermetallic
compound SmNi5
Chem.
Met. Alloys 12 (2019)
77-87
https://doi.org/10.30970/cma12.0396
Vasyl KORDAN, Ivan TARASIUK, Iryna STETSKIV, Roman SERKIZ,
Volodymyr PAVLYUK
The electrochemical
hydrogenation of SmNi5-xMx phases (M = In; In+Sn)
and the binary compound SmNi5 has been studied for the first time by
X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray
spectroscopy, and X-ray fluorescence spectroscopy. All these phases crystallize
in hexagonal CaCu5-type structures. The solubility of In or In+Sn in the binary compound SmNi5 is not higher
than 5.0-5.5 at.%, which can be explained by the large difference between
the atomic radii of the doping components (In, Sn)
and nickel. At charging to the 3 H/f.u. level
(current density 1.0 mA/cm2) the electrodes based on the
binary, ternary and quaternary phases demonstrated maxima of 2.41, 2.52, and
2.72 H/f.u., respectively. The most
geometrically advantageous sites in the initial structures are octahedral voids
(
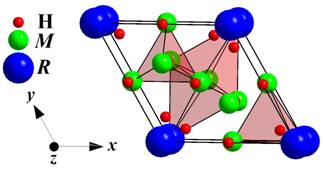
Unit cell of RM5 hydrides and coordination polyhedra
of the H-atoms.
Keywords
X-ray diffraction /
Electron microscopy / Solid solutions / CaCu5-type structure / Ni-MH
battery / Electrochemical hydrogenation