Effect of
element substitution on hydrogen absorption in compounds with the structure type W2CoB2
Chem.
Met. Alloys 12 (2019)
16-20
https://doi.org/10.30970/cma12.0386
Khrystyna MILIYANCHUK, Ladislav HAVELA, Roman GLADYSHEVSKII
Two new compounds, DyErNi2Al and ErLuCoNiAl, with the orthorhombic structure type W2CoB2
(Pearson symbol oI10, space group Immm) were
synthesized. The intermetallics absorbed 5.2 hydrogen
atoms per formula unit at room temperature under a hydrogen pressure of
570 mbar. The hydrogenation resulted in strongly anisotropic lattice
expansion, prevailing along the b‑axis
and accompanied by lattice contraction along the a‑axis, leading to a relative volume expansion of 21.2 %
for DyErNi2AlH5.2 and 17.8 % for ErLuCoNiAlH5.2.
It is shown that element substitution in the positions of both f‑ and d‑elements should be taken into account while tuning the
hydrogenation properties of intermetallics.
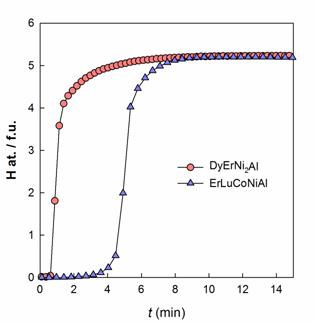
Hydrogen absorption by DyErNi2Al
and ErLuCoNiAl at room temperature as a function of
time.
Keywords
Intermetallics / Metal hydrides / Crystal
structure