Electrochemical lithiation of the CeNiC2 compound
Chem.
Met. Alloys 12 (2019)
9-15
https://doi.org/10.30970/cma12.0384
Vasyl KORDAN, Mykola HEMBARA, Volodymyr PAVLYUK, Bogdan KOTUR
Electrochemical lithiation
of CeNiC2 was carried out in a Swagelok-type
cell with a positive electrode containing LiCoO2 over 51/50 cycles
of lithiation/delithiation. The crystal structure of
CeNiC2 (structure type CeNiC2, Pearson symbol oS8, space group Amm2) before and after lithiation was
investigated by X-ray diffraction. The electrodes containing the ternary
carbide were examined by scanning electron microscopy and energy-dispersive
X-ray spectroscopy. The crystal structure of CeNiC2 remained very
stable toward electrochemical lithiation. The volume
of the unit cell after 51/50 lithiation/delithiation
cycles decreased by only 0.06 %. A small amount of lithium, up to 0.115 Li
per formula unit, was reversibly incorporated into CeNiC2. The
electrochemical lithiation process included two
reactions: 1) insertion of Li into suitable voids and channels of near-surface
layers of CeNiC2, and 2) partial decarbonization
of the electrode. The composition and morphology of the grains of the electrode
material changed only slightly. Small amounts of LizCy, Ce22.7Ni21.6O55.7
and Li2O were detected as by-products of the lithiation.
The lithiation mechanism of ternary carbides
containing a rare earth and a 3d-element
depends on the stoichiometry and peculiarities of the
crystal structure.
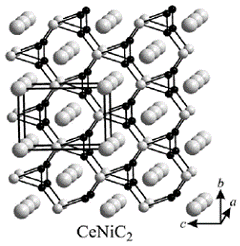
Crystal structure of CeNiC2.
Keywords
Ternary
carbides / X-ray diffraction / Crystal structure / Electrochemical lithiation