Dependence of hydrogen
sorption-desorption on the structure of amorphous and
amorphous-crystalline Fe62.7Nd33.7B1.1Cu1.5Ti1.0 alloys
Chem.
Met. Alloys 11 (2018)
85-91
https://doi.org/10.30970/cma11.0372
Gregory BREKHARYA, Tetyana PRYADKO, Volodymyr DEKHTYARENKO, Vasyl LYAKISHEV, Nataliya LYASHENKO, Vira BOVDA
Thin films of Fe62.7Nd33.7B1.1Cu1.5Ti1.0
alloy, promising for use as permanent magnets with high values of HÑj
and Br, were
obtained by quenching from the liquid state in pure helium atmosphere. The
sorption properties and kinetics parameters of the hydrogenation and
dehydrogenation processes of amorphous and amorphous-crystalline alloys were
investigated by Sieverts’ method. The temperature of
onset of intensive hydrogen absorption was determined by the kinetic dependence
T = f(t), p = f(t). The amount of absorbed hydrogen mH [g]
and the hydrogenation rate change v [g/s]
in the process of hydrogen saturation were calculated from the pressure change
in a closed volume. It was established that the hydrogenation process is
controlled by the degree of amorphousness of the obtained thin films. The more
amorphous the component, the slower the hydrogen absorption, and it occurs at higher temperatures. It was found that at ~790±5 K the amorphous component decomposes into an equilibrium of Nd,
Fe14Nd2B, and Fe4Nd1,1B4
phases in both types of film and precipitated Nd
takes part in the hydrogenation process at this temperature. The coexistence of
two metal hydride phases possessing different properties is possible in
partially hydrogenated films and their volume ratio can be regulated by
appropriate selection of the sorption-desorption
process parameters. The hydrogen brittleness of the samples may be used to
obtain microstructured films with a grain size of
50-300 nm.
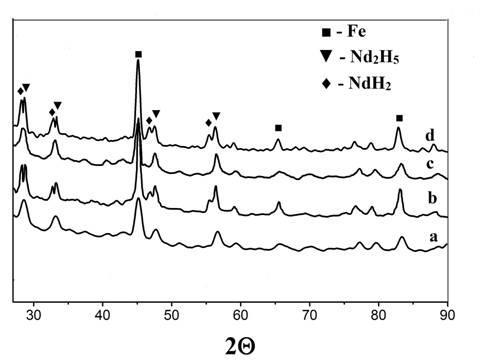
X-ray
diffraction patterns of hydrogenated (à, c) and dehydrogenated (b, d) Fe62.7Nd33.7B1.1Cu1.5Ti1.0
alloy films (à, b – 1st cycle, c, d – 2nd cycle).
Keywords
Permanent magnets / Amorphous-crystalline state
/ Hydrogenation-dehydrogenation process / Disproportionation
/ Hydride phases