Phase equilibria
in the ternary system Gd–Fe–Zn and electrochemical hydrogenation of the phases
Chem.
Met. Alloys 11 (2018) 27-33
https://doi.org/10.30970/cma11.0381
Nataliya Chorna, Nataliya Sagan, Oksana Zelinska,
Vasyl Kordan, Anatoliy Zelinskiy, Volodymyr Pavlyuk
The interaction of the components in the
ternary system Gd–Fe–Zn was investigated for the first
time using X-ray powder diffraction and energy-dispersive X-ray analysis. The
isothermal section of the phase diagram at 500°C was constructed over the whole
concentration range. The existence of the ternary compound Gd2Fe2Zn15
(Th2Zn17-type, space group R‑3m, à = 0.90025(10) nm, ñ = 1.3160(3) nm) was
confirmed at 500°C in the Gd–Fe–Zn system. A new ternary compound ~Gd13(Fe,Zn)58 (Gd13Zn58-type,
space group P63/mmc, à = 1.4306(5) nm, ñ = 1.4000(2) nm)
was obtained. The efficiency of electrochemical
hydrogenation of the binary phases GdZn, GdZn2,
GdFe2 and the solid solutions on their
basis was studied in Ni‑MH battery prototypes.
GdZn1‑xFex with cubic CsCl-type structure reversibly absorbs 0.006 Í/f.u. when x = 0, and 0.009 H/f.u. when x = 0.04. GdZn2‑xFex with orthorhombic KHg2-type structure
absorbs 0.012 Í/f.u. when x = 0, and 0.016 H/f.u. when x = 0.06. GdFe2‑xZnx with cubic MgCu2-type structure absorbs the
largest amount of hydrogen, that is 0.023 Í/f.u.
when x = 0, and
0.027 H/f.u. when x = 0.06.
The influence of composition, crystal structure and chemical activity of the
studied electrode materials in alkaline media on their hydrogen capacity and
electrochemical characteristics is discussed.
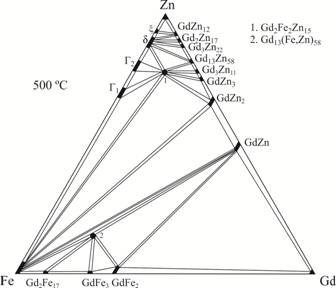
Isothermal section of the Gd–Fe–Zn phase diagram at 500°Ñ.
Keywords
Phase equilibria / Intermetallic
compound / Solid solution / Electrochemical
hydrogenation / Ni‑MH battery