Influence of doping elements
on the electrochemical hydrogenation efficiency of Tb2Ni17-based
phases
Chem.
Met. Alloys 10 (2017)
61-68
https://doi.org/10.30970/cma10.0355
Vasyl KORDAN, Vitaliy NYTKA, Grzegorz KOVALCZYK, Agnieszka
BALINSKA, Oksana ZELINSKA, Roman SERKIZ, Volodymyr PAVLYUK
Electrochemical hydrogenation of the phases Tb2Ni17-xMx, x ≈ 1
(M = Li, Mg, Al, Ge, Sn, Sb,
Bi, Co) that crystallize in the Th2Ni17-type structure
was investigated for the first time. The phases
containing the s‑element Li
or Mg, or a mixture of these (Li, Mg) as doping element showed the best Coulomb
efficiency. Under the conditions of the experiment (10 mA·h
charge) pure Tb2Ni17 absorbed approximately 0.67 H/f.u.
(50.0 % efficiency), the Li-containing phase approximately 1.43 H/f.u.
(86.0 %), the Mg-containing phase 1.37 H/f.u.
(76.3 %), and the phases with a mixture of Li and Mg, namely Tb2Ni16Li0.4Mg0.6
and Tb2Ni15.6Li0.6Mg0.8,
1.46 H/f.u. (91.5 %) and 1.50 H/f.u. (95.0 %), respectively. The
phases with p-elements such as Al, Ge, Sn revealed interaction of the surface with the electrolyte, but showed
structural and corrosion stability over 30 charge-discharge cycles. The
Al-containing phase absorbed 1.09 H/f.u. (67.8 %
efficiency), the Ge-containing phase 1.05 H/f.u. (63.1 %), the Sn-containing phase 0.76 H/f.u. (52.5 %). the Sb‑containing phase
1.24 H/f.u.
(75.5 %), and the Bi-containing phase
1.46 H/f.u.
(79.8 %). Cobalt was added to the initial binary compound in larger amounts
(up to 26.3 at.%)
because smaller quantities did not increase the amount of absorbed
hydrogen, but even high Co contents did not affect the results significantly
(0.98 H/f.u., 59.5 % efficiency). In all
cases intercalation of hydrogen occurred in octahedral voids (Wyckoff position
6h) of the initial structures, i.e. the coordination polyhedron of the
H-atoms was an octahedron [HTb2(Ni,M)4]. Electron microprobe
analysis showed that the electrodes on the basis
of Tb2Ni17‑xMx were stable in the
electrolyte over 30-50 cycles of electrochemical processes. Cyclic voltamperometry, impedance measurements and corrosion
studies of the electrode materials also confirmed their stability in
alkaline solutions of electrolyte (6 M KOH).
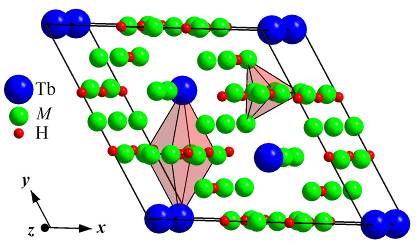
The unit cell of Tb2Ni17-xMxHy hydrides.
Keywords
Intermetallic compound / Solid solution / Electrochemical hydrogenation / Electrode material /Ni-MH battery