Decarbonization of the Ce2Mn17C1.77 compound upon lithiation
Chem.
Met. Alloys 10 (2017)
45-49
https://doi.org/10.30970/cma10.0352
Mykola HEMBARA, Vasyl
KORDAN, Volodymyr PAVLYUK, Bogdan
KOTUR
The ternary
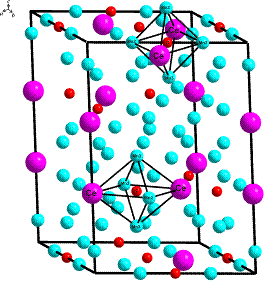
compound Ce2Mn17C1.77 was investigated by
means of X-ray powder diffraction, scanning electron microscopy and
energy-dispersive X-ray analysis. The structure type Pr2Mn17C1.77
was confirmed using the
Rietveld method. Electrochemical lithiation of Ce2Mn17C1.77
was carried out in Swagelok-type cells with a
positive electrode containing LiCoO2 over 30 cycles. Decarbonization of the Ce2Mn17C1.77
ternary carbide upon lithiation was accompanied by a
decrease of the cell volume (from 847.9(2) Å3 to
842.7(5) Å3) and formation of α-Li2C2,
LixCy carbides, and amorphization of the Ce2Mn17 subcell. The morphology of the anode surface underwent
changes and the grain size of the material decreased.
Keywords
Carbides
/