Electrochemical hydrogenation
of Tb2Ni17-xMx (M = Mg, Sn) phases
Chem.
Met. Alloys 9 (2016) 153-157
https://doi.org/10.30970/cma9.0346
Vasyl KORDAN, Oksana ZELINSKA, Volodymyr PAVLYUK, Vitaliy
NYTKA, Roman SERKIZ
The electrochemical hydrogenation of Tb2Ni17-xMx phases (M =
Mg, Sn) was studied for the first time. Both compounds have the hexagonal Th2Ni17-type
structure and small homogeneity ranges, x = 0-1
for TbNi17-xSnx and x = 0-1.5 for TbNi17-xMgx.
Under the conditions of the experiment pure Tb2Ni17
absorbed approximately 0.55 H/f.u. The
Mg-containing phase absorbed approximately 1.14 H/f.u. and the
Sn-containing phase ~0.63 H/f.u. In all cases intercalation of hydrogen
occurred in octahedral voids 6h of
the initial structures, so the coordination polyhedron of the H-atom is an
octahedron [HTb2M4].
The Tb2Ni17-xMgx phase absorbed the largest
amount of hydrogen because magnesium, like rare-earth and transition metals, is
able to absorb hydrogen and a combination of these elements leads to better hydrogen
absorption. Electron microprobe analysis showed that the
electrodes on the basis of Tb2Ni17-xMx were
stable in the electrolyte during the electrochemical processes, i.e. the qualitative and quantitative
composition of the observed phases remained unchanged.
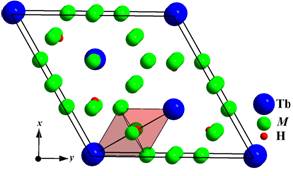
Unit cell of Tb2Ni17-xMxHy
(y < 3) hydrides and
coordination polyhedron of the H-atom.
Keywords
Intermetallic
compound / Ni-MH battery / Electrochemical hydrogenation