Thermodynamic and
potentiometric investigation of the surface processes on Hg3In2Te6
electrodes
Chem.
Met. Alloys 9 (2016)
123-127
https://doi.org/10.30970/cma9.0341
Oxana SEMA,
Volodymyr DIICHUK, Igor KOBASA
The influence of
chemical etching and preliminary cathodic and anodic polarization on the
stationary electrode potential of a Hg3In2Te6
electrode has been investigated in various acidic solutions with different
pH-values. It is shown that In3+ ions govern the process of
formation of a double electric layer at the interphase
semiconductor/electrolyte for electrodes submitted to preliminary chemical
etching and anodization, while Hg2+ ions play the leading role in a
similar process occurring on the electrodes after cathodic polarization.
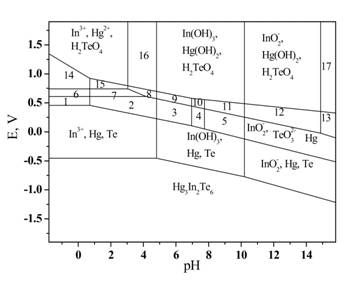
Pourbaix diagram for the system Hg3In2Te6–H2O
(С = 10-6
mole/l).
Keywords
Hg3In2Te6
/ Pourbaix diagram / Thermodynamic analysis / Cathodic and anodic polarization