Electrochemical lithiation of Ti5M3, Ti3M and Zr3M (M = Sn, Sb) binary intermetallics
Chem.
Met. Alloys 9 (2016)
84-91
https://doi.org/10.30970/cma9.0327
Vasyl KORDAN, Oksana ZELINSKA, Volodymyr PAVLYUK, Igor OSHCHAPOVSKY, Roman
SERKIZ
The binary phases Ti5M3, Ti3M and Zr3M (M = Sn, Sb) were studied for electrochemical
lithiation, using powder X-ray diffraction, scanning
electron microscopy (SEM) and energy-dispersive X-ray
analysis (EDX). The investigation showed that the
morphology of the cathode and the anode surfaces undergo changes, and the grain
size of the materials decreases. The phase analysis of the anode materials
revealed that the Ti5Sn3 (structure type Mn5Si3)
and Ti3Sn (structure type Mg3Cd) phases form solid
solutions by insertion of Li atoms into the initial structure. The insertion is
reversible. The phases Ti5Sb3 (structure type Y5Bi3),
Ti3Sb, Zr3Sn (structure type Cr3Si), and Zr3Sb
(structure type Ni3P) form solid solutions by substitution of Li for
Sn or Sb atoms. Only the Zr3Sb
phase showed weakly reversible substitution. Among the investigated compounds,
the most suitable structure types for intercalation of lithium appeared to be
the Mn5Si3- and Mg3Cd-types, where the Li
atoms occupy octahedral voids. The intermetallic
compounds containing tin showed better ability for electrochemical lithiation than the compounds containing antimony. This can
be explained by the easier interaction of antimony and lithium with the
formation of binary compounds.
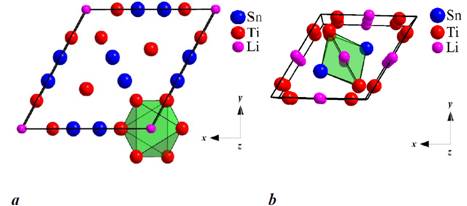
Projection of the unit cells of Ti5Sn3Lix (a)
and Ti3SnLix (b).
Keywords
Intermetallic compound / Electrochemical
lithiation / Li-ion battery