The effect of 4 wt.% Cu addition on the electrochemical corrosion behavior of
automotive engine Al-6Si-0.5Mg alloy
Chem.
Met. Alloys 8 (2015) 69-74
https://doi.org/10.30970/cma8.0307
Abul HOSSAIN, Fahmida GULSHAN, A.S.W. KURNY
The purpose of this paper was to investigate
the effect of 4 wt.% Cu addition on the
electrochemical corrosion behavior of Al-6Si-0.5Mg alloy in NaCl
solution. The corrosion of thermally treated samples was characterized by
electrochemical techniques. On the whole, the electrochemical test showed that
Cu addition increases the corrosion rate of the alloy. The magnitude of the
noble shift in the open circuit potential (OCP),
corrosion potential (Ecorr)
and pitting corrosion potential (Epit) increased with the addition of 4 wt.% Cu to the Al-6Si-0.5Mg alloy.
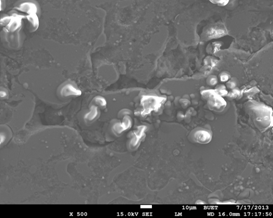
SEM Secondary Electron Image of the damaged
surface morphology of the Al-6Si-0.5Mg-4Cu alloy in 0.1M NaCl
solution (immersion time ~67 min at room temperature).
Keywords
Al-6Si-0.5Mg-4Cu alloy / Electrochemical
test / SEM