On the preparation, structure and bonding of
ReSCl3
Chem.
Met. Alloys 8 (2015) 43-54
https://doi.org/10.30970/cma8.0306
S.V. VOLKOV,
V.V. SUBBOTIN, P.Yu. DEMCHENKO, R.E. GLADYSHEVSKII, O.G.
YANKO, L.B. KHARKOVA
The reaction of Re2O7
with a solution of S in S2Cl2
at 100°C produced rhenium sulfur trichloride, ReSCl3. The
compound was found to have
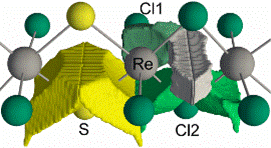
a polymeric linear structure [{ReCl2(μ-Cl)}2(μ-S)2]∞,
which represents a new structure type
(space group C2/m,
a = 11.4950(7), b = 6.5626(3), c =
5.9938(4) Å, β =
95.199(4)°, Z = 4). The structure is
closely related to the NbCl4-type and expands the
series of similar one-dimensional chain structures. Distorted Re[Cl4S2]
octahedra share edges to form
infinite straight chains along the
[010] direction and the Re atoms
are arranged in pairs with
alternatively short (2.965
Å) and long (3.598
Å) Re–Re distances. Electronic
structure calculations and chemical bonding
analysis, using the electron
density/electron-localizability indicator (ELI) approach, indicated overall metallic character and revealed a combination of covalent polar Re–Cl and Re–S bonds and weak Re–Re non-polar metallic bonds, yielding the ELI-based oxidation
numbers (ELIBON) Re+3.40S–1.20[Cl1–0.68]2Cl2–0.84.
The reaction of Re2O7
with a solution of S in S2Cl2
at 100°C gave ReSCl3,
a polymer exhibiting an one-dimensional chain structure [{ReCl2(μ-Cl)}2(μ-S)2]∞,
with covalent polar Re–Cl, Re–S bonds and weak Re–Re non-polar metallic bonds.
Keywords
Rhenium sulfidochlorides
/ Crystal structure / One-dimensional chain structures / Density functional calculations / Electron localizability indicator / Chemical bonding