Catalytic activity of Pd(II) and Cu(II) complexes anchored to natural and
pre-modified bentonite in the oxidation of carbon
monoxide
Chem.
Met. Alloys 8 (2015) 32-38
https://doi.org/10.30970/cma8.0301
T.L.
RAKITSKAYA, V.O. VASYLECHKO, T.A. KIOSE, G.V. GRYSHCHOUK, A.M. DZHIGA, V.Y. VOLKOVA
The influence of different methods of
pre-modification (thermal, hydrothermal, and acid-thermal) of natural bentonite on the catalytic activity of bentonite-anchored
palladium(II) and copper(II) complexes in low-temperature
oxidation of carbon monoxide has been studied. The contributions of weakly and
strongly bound Pd(II) and Cu(II) forms to the total
activity of the catalysts were evaluated by two methods, desorption
of the metal ions and a kinetic method.
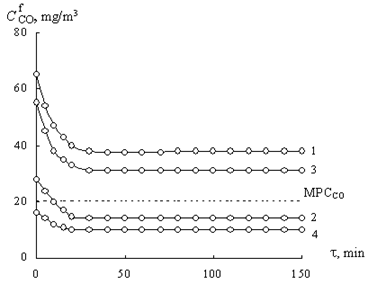
Time dependence of the
concentration of CO during the oxidation of carbon monoxide with oxygen over Pd(II)‑Cu(II) catalysts based on natural (curve 1) and pre-modified bentonite (curves 2-4).
Keywords
Desorption
of palladium(II) and copper(II) / Natural and
pre-modified bentonite / Catalysts for carbon monoxide
oxidation