Yb2Al15Pt6
- the
most ordered variety of the Sc1.2Fe4Si9.8 aristotype
Chem.
Met. Alloys 7
(2014) 85-99
https://doi.org/10.30970/cma7.0276
Yurii Prots, Micha Deppe, Raul Cardoso-Gil, Antonio Cervellino, Alim
Ormeci,
Christoph Geibel, Yuri Grin
The title compound was synthesized by reaction
of the elemental components in a corundum crucible at 1450 ºC, and
subsequent crystal growth using the Bridgman technique. The crystal structure
of Yb2Al15Pt6 was determined from X-ray single
crystal diffraction data: space group Cmcm, Pearson symbol oS92,
a = 12.7969(1) Å, b = 7.38813(7) Å, c = 16.3605(2) Å, RF = 0.036 for 1455 observed structure
factors and 65 variable parameters. The title compound crystallizes in a new
type of structure, which represents an ordered variety of the Sc1.2Fe4Si9.8
aristotype. The structure can be described as an (ABB)2 stacking of two trigonal
layers with compositions Yb4Al6 (A) and Pt6Al12 (B). The layers A are almost
planar and consist of ytterbium atoms arranged at the apexes of condensed
hexagons centered by triangular Al3 units. The slabs B may be considered as distorted hexagonal close-packed, similar to
three adjacent layers I-Cd-I of the structure type CdI2 (Al-Pt-Al). The structural particularities
of Yb2Al15Pt6 are discussed in comparison with
the related structures of Y2Ga9Co3, Tb2Ge3Pt9,
Yb2Ga9Pd3 and Er4Al24Pt9.
A real-space analysis of the chemical bonding with the electron-localizability
approach showed that the crystal structure of Yb2Al15Pt6
consists of an anionic Al-Pt framework, with Yb cations embedded in cavities.
The Al-Pt interactions within the framework
are covalent polar, whereas ionic bonding is observed between ytterbium and the
framework.
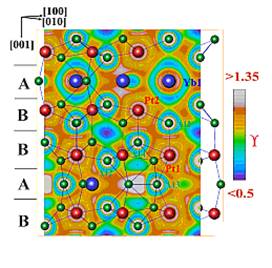
Electron localizability indicator
for Yb2Al15Pt6.
Keywords
Ytterbium /
Platinum / Aluminum / Crystal structure / Intermetallic
compound