Thermodynamics and crystal chemistry of the RE2MgNi9H12-13 (RE = La and Nd) hydrides
Chem.
Met. Alloys 7 (2014) 1-8
https://doi.org/10.30970/cma7.0243
Volodymyr YARTYS, Roman DENYS
Ternary RE-Mg-Ni
intermetallics are promising negative electrode materials for
high-energy/high-power Nickel-Metal Hydride (Ni-MH) batteries. These compounds
belong to a family of hybrid layered structures (AB3, A2B7 and A5B19;
A = RE, Mg; B = Ni),
composed of stacked Laves-type layers, RE2‑xMgxNi4, and Haucke-type RENi5 layers. In the present study structural and
hydrogen storage properties of a new compound, Nd2MgNi9
(PuNi3 type; a = 4.9783(1),
c = 24.1865(6) Å),
are reported and compared with those of the isostructural La2MgNi9
intermetallic. RE2MgNi9
(RE = La and Nd) were found
to easily form hydrides containing 13 (La) or 12 (Nd) H/f.u. As for La2MgNi9H13,
formation of the Nd2MgNi9H12 hydride proceeds
via isotropic expansion of the unit cell (a = 5.3234(2),
c = 26.506(2) Å;
ΔV/V = 25.3 %). In
situ neutron diffraction studies of the saturated deuterides La2MgNi9D13
and Nd2MgNi9D12, performed at SINQ, PSI,
Switzerland, revealed: (a) nearly equal distribution of H atoms within the REMgNi4 and RENi5 layers; (b) preferred
filling of the Mg- and Ni-surrounded sites within the REMgNi4 layers; (c) local hydrogen ordering with the
H-sublattice built from stacking of MgH6 octahedra and NiH4
tetrahedra, indicating directional metal–hydrogen bonding. In spite of the
similarity of the crystal structures and hydrogenation capacities, Nd2MgNi9H12
shows a significantly lower thermodynamic stability (DHdes = 29 kJ/mol H2)
than La2MgNi9H13 (DHdes = 36 kJ/mol H2).
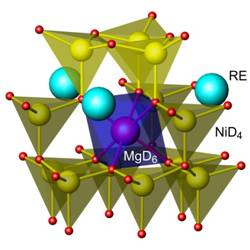
Hydrogen sublattice in the structures RE2MgNi9D12-13deuterides,
formed by stacking of the MgD6octahedra and NiD4tetrahedra
Keywords
Metal
hydrides / Magnesium / Neodymium / Nickel / Powder neutron diffraction /
Crystal structure / Hydrogen