Thermal solid-phase
transformations of hydrated Zn-Co(II) diphosphates
Chem.
Met. Alloys 7
(2014) 9-14
https://doi.org/10.30970/cma7.0236
N.M. ANTRAPTSEVA, N.V. SOLOD, L.B. KOVAL, T.S. GRYSYUK
The sequence of physico-chemical
and structural transformations occurring
during the thermolysis of the solid solution
of diphosphates of composition Zn2-xCoxP2O7∙5H2O (0 ≤
õ ≤ 0.69) has been established.
The composition, temperature ranges of formation and the thermal stability of
the products of partial and complete dewatering have been determined. The final
thermolysis product, the solid solution of anhydrous diphosphates α-Zn2-xÑîxP2O7
(0 ≤ õ ≤ 0.69), has been
identified. It is shown that the formation proceeds via two routes. The first
route consists in thermal dehydration of the original diphosphate
(up to 57-63 %). Along the second route, up to 43-37 % α-Zn2-xÑîxP2O7 is formed by solid-phase
reaction of partial dehydration products. A general scheme of the solid-phase
thermal transformations of Zn2-xÑîxP2O7∙5H2O
(0 ≤ õ ≤ 0.69) is
proposed.
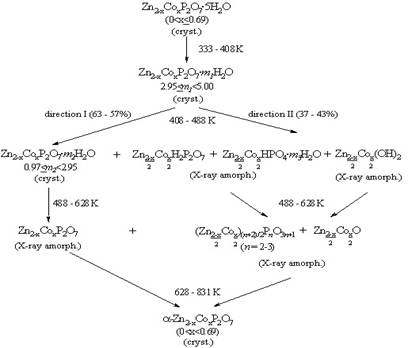
Reaction scheme for the dehydration of Zn1.31Ñî0.69Ð2O7∙5H2O
Keywords
Thermolysis
/ Thermal dehydration / Destruction / Diphosphates