Synthesis and catalytic properties of Mg, Co(II),
Zn phosphate solid solutions
Chem.
Met. Alloys 6
(2013) 70-74
https://doi.org/10.30970/cma6.0242
N.M. ANTRAPTSEVA, N.V. SOLOD, L.B. KOVAL
Dihydrogenphosphates with the general formula Zn1-õÑîx(H2ÐO4)2·2Í2Î (0 < õ < 1)
and phosphates of composition Co3-õMgõ(PO4)2·8Í2Î (0 < õ ≤ 1)
were synthesized. The former crystallize
in the monoclinic system, space group Ð21/n
(Z = 2), with the formation of a continuous substitutional solid solution. The latter form a limited
solid solution. Their catalytic properties were investigated. The effectiveness
of their action as catalysts for alcohol dehydration and methane oxidation was
shown.
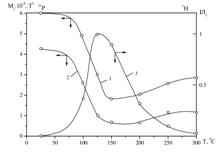
Interrelation of results of heat
treatment of Zn0.5Ñî0.5(H2PO4)2·2H2O
at different temperatures as obtained by proton NMR and 31Ð NMR (1 –
width and 2 – second moment of lines in the 31Ð NMR
spectra; 3 – intensity of the narrow component in the 1H NMR spectra)
Keywords
Phosphate / Solid solution / Synthesis /
Catalyst