Electrochemical synthesis of
lithium fullerides
Chem.
Met. Alloys 6
(2013) 40-42
https://doi.org/10.30970/cma6.0238
A.O. ZUL’FIGAROV, V.A. POTASKALOV, A.P. POMYTKIN, A.A. ANDRIIKO, D.V. SHCHUR, O.A.
KRIUKOVA, V.G. KHOMENKO
The
electrochemical reaction of Li with fullerene in an aprotic
electrolyte was studied. It was found that a lithium-rich fulleride
with approximate formula Li10C60 can be obtained in the
presence of a catalyst. This amount of Li is irreversibly incorporated into the
structure. Additionally, 4-5 atoms can be introduced reversibly, thus allowing
cycling the material with an initial reversible capacity of about 180 mAh·g-1.
The pyrolysis product of a heterometal
complex with monoaminoethanol ligands
is shown to be a good catalyst for this process.
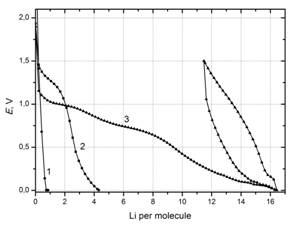
Galvanostatic curves of Li+ reduction
on fullerene C60 at a specific current of 40 mA·g-1.
1 – initial
sample; 2 – fullerene with grafted 2Co-Ni complex with monoethanol amine;
3 – the same
with the complex pyrolized in argon at 500°C
Keywords
Fullerene / Lithium fulleride
/ Electrochemical synthesis