Copper(I)
complexes with 3,3′-iminodipropanenitrile
and 3-(diallylamino)propanenitrile: synthesis and crystal structure of σ-[{(NH(H+)(C2H4CN)2}Cu3Cl4]
and π-,σ-[Cu((C3H5)2NC2H4CN)ClO4]
compounds
Chem.
Met. Alloys 5
(2012) 173-180
https://doi.org/10.30970/cma5.0227
Mykhaylo LUK’YANOV, Evgeny GORESHNIK, Oleksiy PAVLYUK, Maryan MYS’KIV
Starting from ethanolic
or water-propanolic solutions of 3,3′-iminodipropionitrile
(L1), 3-(diallylamino)propanenitrile (L2),
and CuCl2·2H2O or Cu(ClO4)2·6H2O,
respectively, two complexes, [(NH(H+)(C2H4CN)2Cu3Cl4]
(I) and [Cu((C3H5)2NC2H4CN)ClO4]
(II), were obtained by the alternating current electrochemical technique and
investigated by single crystal X-ray diffraction. The crystal structures of I
and II are monoclinic, for I: space group P21/n, a
= 8.9700(2), b = 14.3951(4), c = 10.0156(3) Å,
β = 94.056(1)°, V = 1290.02(6) Å3, Z = 4; for II: space group Cm, a
= 10.0916(8), b = 8.1061(7), c = 8.4207(7) Å,
β = 116.581(5)°, V = 616.03(5) Å3, Z = 2. The coordination of the Cu(I) atoms in complex I includes three Cl atoms and the N atom
of a cyanogroup. Cations H+L1 connect separate corrugated
layers (Cu3Cl4–)n into a framework.
The coordination environment of the copper(I)
atom in complex II is formed by
two C=C bonds of the allyl groups of one molecule L2, the nitrile
N atom of another molecule L2, and
the apical O atom of a ClO4 anion. The inorganic anions ClO4–
also interconnect the metalorganic ribbons via
O...H-C hydrogen bonds.
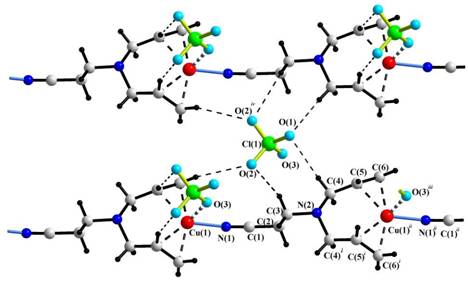
Projection of the
structure of [Cu((C3H5)2NC2H4CN)ClO4]
Symmetry
codes: (i) x, −y, z; (ii)
−1+x, y, −1+z;(iii) −1/2+x,
−1/2+y, −1+z; (iv) x, 1−y, z.
Keywords
Copper(I) / π- and
σ-Complexes /