Determination of ruthenium(III) in the presence of micellar medium by derivative spectrophotometric technique
Chem.
Met. Alloys 5 (2012) 42-49
https://doi.org/10.30970/cma5.0207
M. RAMESWARA RAO,
K.B. CHANDRASEKHAR, N.
DEVANNA
A sensitive spectrophotometric
method has been developed for the determination of ruthenium(III) using 3,5‑dimethoxy‑4‑hydroxybenzaldehyde
isonicotinoylhydrazone (DMHBIH) in presence of neutral miceller medium of
Triton X‑100. The metal ion forms a brownish yellow coloured water
soluble complex with the DMHBIH
reagent in acidic buffer pH 4.25. Spectral data shows the formation of an 1:1 (M:L) complex with a
stability constant 1.82 × 106.
Beer’s law is obeyed
over a concentration range of 0.202 to 4.043 μg/mL of Ru(III) at
390 nm. The molar absorptivity and Sandell’s sensitivity are calculated to be 1.7 × 104 L.mol‑1.cm‑1
and 0.006 μg.cm‑2 Ru(III) respectively. The
developed derivative spectrophotometric method was employed for the
determination of Ru(III) in synthetic alloy samples and real water samples. The
effect of various diverse ions has also been studied.
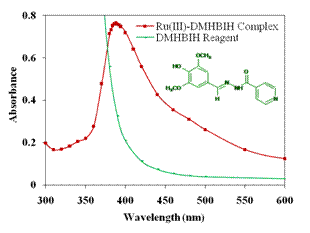
Typical Zero order Absorption Spectra of Ru(III)-DMHBIH complex Vs
Reagent as blank
Keywords
Derivative spectrophotometry / Ruthenium(III) / 3,5-dimethoxy-4-hydroxybenzaldehyde
isonicotinoylhydrazone (DMHBIH)
/ Synthetic alloy samples / Real water samples