A new approach to describe elemental-property
parameters
Chem.
Met. Alloys 1
(2008) 1-23
https://doi.org/10.30970/cma1.0007
Pierre VILLARS, Jo DAAMS, Yoshihiro SHIKATA, Krishna RAJAN, Shuichi IWATA
The atomic number (AN) of the elements together with
their ‘periodic number’ (PN) were found to form an efficient pair for the
discussion of metallurgical and structural problems. The periodic number PN
represents a different enumeration of the elements, emphasizing the role of the
valence electrons. In contrast to the atomic number, PN depends in details on
the underlying Periodic Table of the elements. As a first result we describe
the elemental-property parameters ‘atomic size SZa’ and ‘atomic
reactivity REa’, derived from fits to various experimental and
theoretical data sets. These two parameters can be approximated as simple
functions of AN and PN:
SZa = kSZ [log (AN +
1)] [kPN – (log PN)3],
REa = kRE {[log (AN +
1)] [kPN – (log PN)3]}-1
= kSZ kRE (SZa)-1,
where kPN is a scaling factor, and kSZ,
kRE are fit parameters for a fit to experimental data. We argue that
all elemental-property parameter patterns are derived from AN and PN. AN and PN
represent fundamental elemental-property parameters independent from each
other. Any pattern, which shows well-defined functional behavior within each
group number GN, as well as within each main quantum number QN, can be
included. On the example of compound formers/non-formers in binary, ternary and
quaternary chemical systems we demonstrate that a quantitative link exists
between material properties and AN, PN (or simple functions of both) of the
constituent elements.
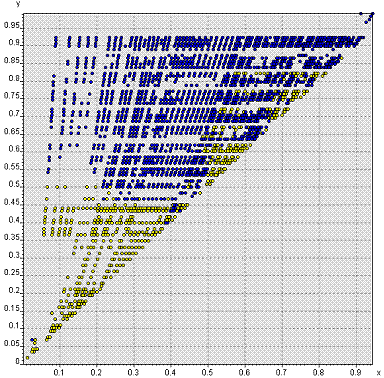
Separation of 2318
binary systems into compound formers and non-formers.
Keywords
Electronegativity / Atomic radii / Chemical
elements